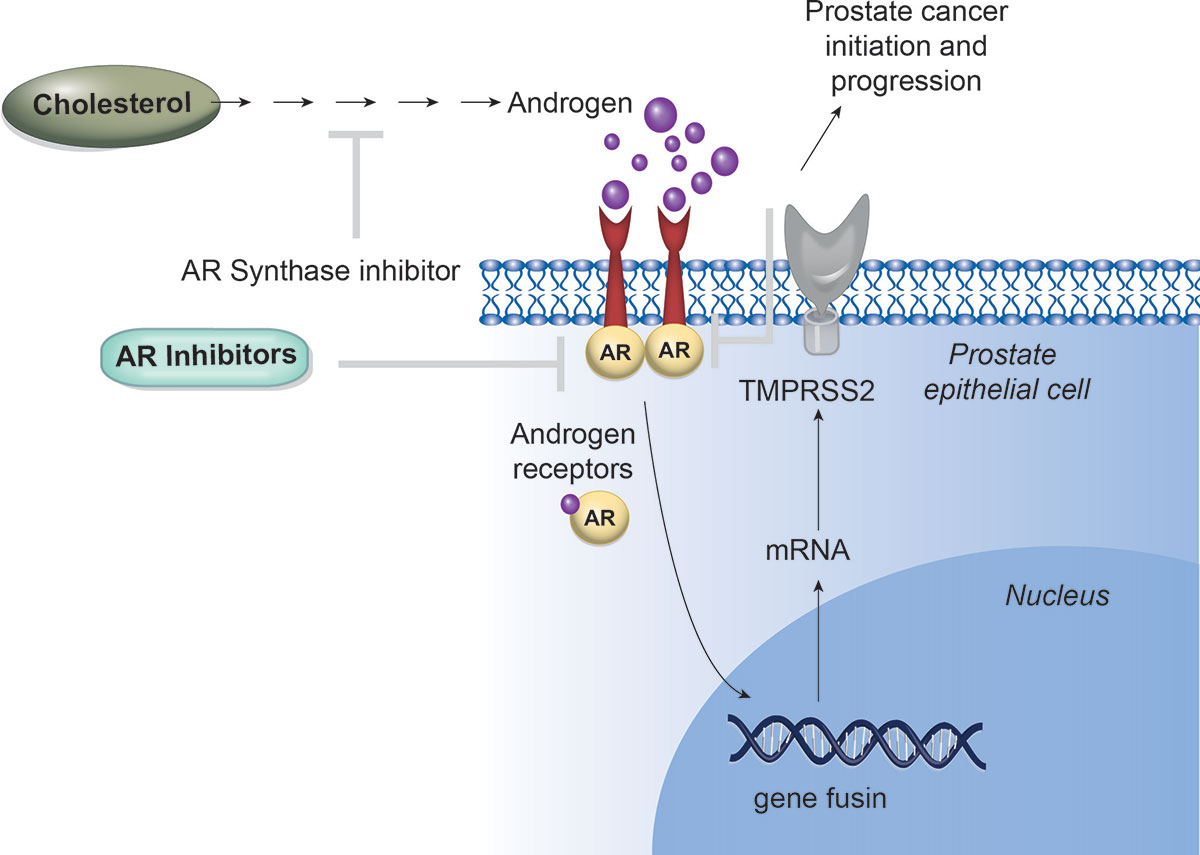
Targeting AR Signaling in Prostate Cancer
The activation of the androgen receptor (AR) and the AR-driven transcriptional programs play a crucial role in the pathophysiology of prostate cancer.
Although targeting AR has seen significant success in translational efforts, therapeutic resistance frequently arises due to molecular changes in the androgen signaling pathway.
The effectiveness of next-generation AR-directed therapies for castration-resistant prostate cancer has clinically validated the ongoing reliance on AR signaling and has expanded the treatment options available for men with both castration-resistant and castration-sensitive forms of the disease.
On November 16, 2023, the Food and Drug Administration approved enzalutamide (Xtandi, Astellas Pharma US, Inc.) for non-metastatic castration-sensitive prostate cancer (nmCSPC) with biochemical recurrence at high risk for metastasis (high-risk BCR).
On June 3, 2025, the Food and Drug Administration (FDA) approved darolutamide (Nubeqa, Bayer Healthcare Pharmaceuticals Inc.) for metastatic castration-sensitive prostate cancer (mCSPC). The FDA previously approved darolutamide in combination with docetaxel for mCSPC.
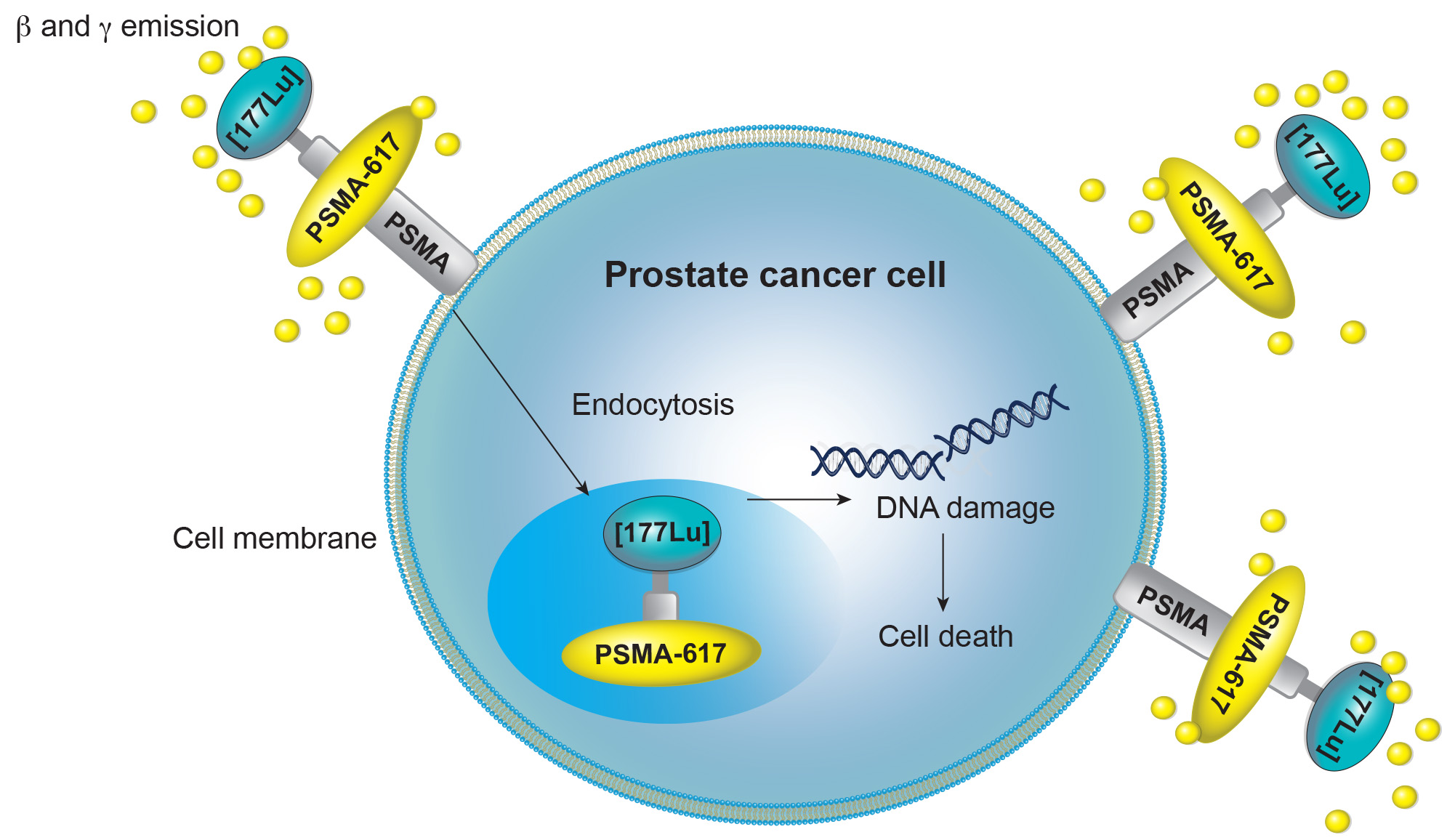
PSMA Targeted Therapy
PSMA, a cell membrane protein highly expressed on prostate cancer cells, is an ideal target for imaging and therapy.
Various PSMA-targeted therapies are in development, including radioligand therapies (177Lu-PSMA-RLT, 225Ac-PSMA-RLT), antibody-drug conjugates, cellular immunotherapies (CAR-T, CAR/NK-92, PSMA-targeted BiTE), photodynamic therapy, imaging-guided surgery (radionuclide, fluorescence, multimodal), and ultrasound-mediated nanobubble destruction.
The first PSMA-targeted therapy, LuPSMA, was approved in 2022 for patients with PSMA-positive mCRPC.
On March 28, 2025, the Food and Drug Administration expanded the indication for lutetium Lu 177 vipivotide tetraxetan (Pluvicto, Novartis Pharmaceuticals Corporation) to include adults with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor pathway inhibitor (ARPI) therapy and are considered appropriate to delay taxane-based chemotherapy.
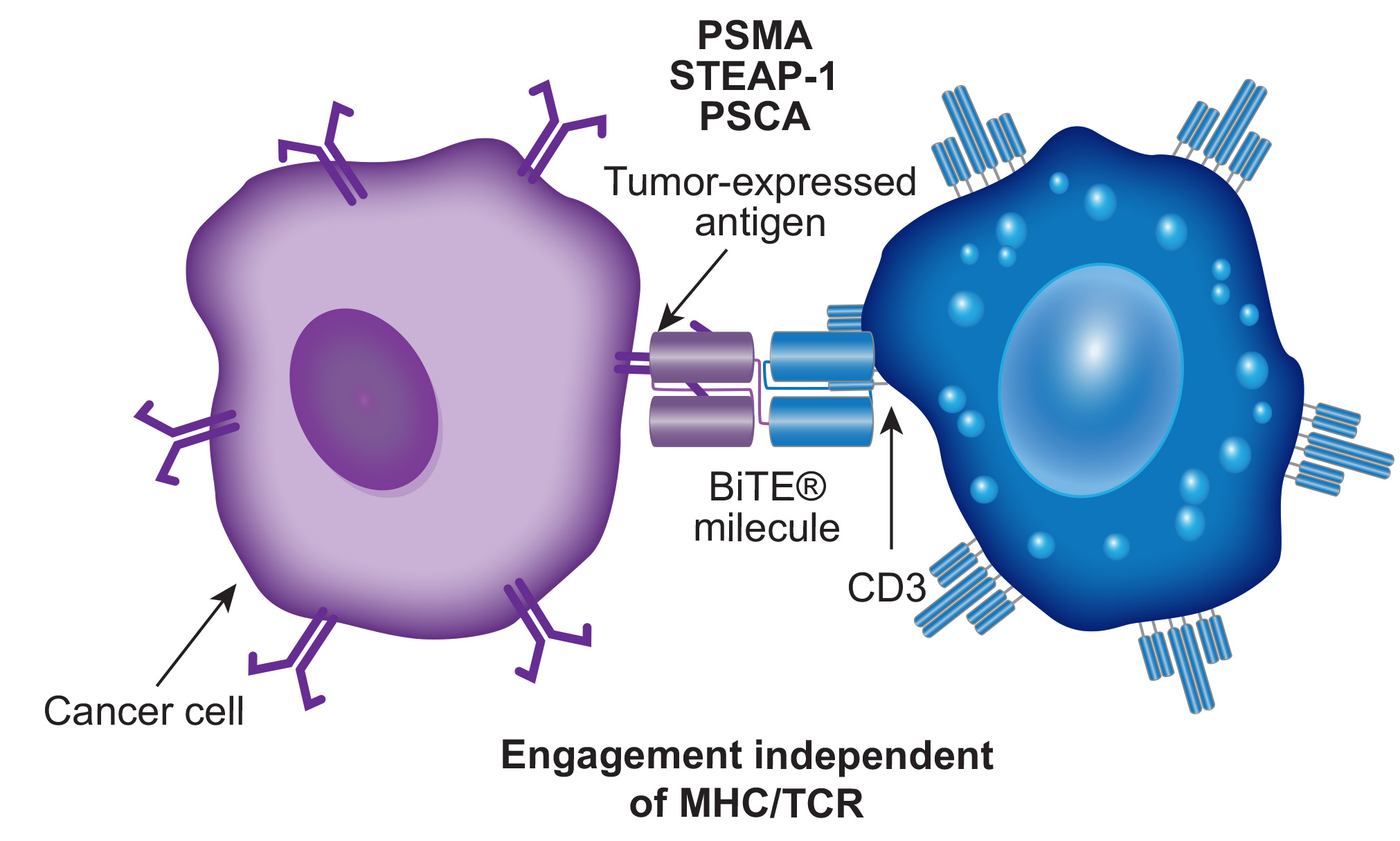
BiTEs in Prostate Cancer
Immune checkpoint inhibitors have shown limited effectiveness in a small subset of prostate cancer patients and have not significantly impacted advanced cases.
The identification of prostate-specific membrane antigen (PSMA) as a specific tumor-associated antigen has renewed interest in immunotherapies for prostate cancer.
T-cell immunotherapies, such as bispecific T-cell engagers (BiTEs) and chimeric antigen receptor (CAR) T-cell therapy, which have been highly successful in treating hematologic cancers, are now being tested for prostate cancer, targeting ligands like PSMA, STEAP1, and PSCA.
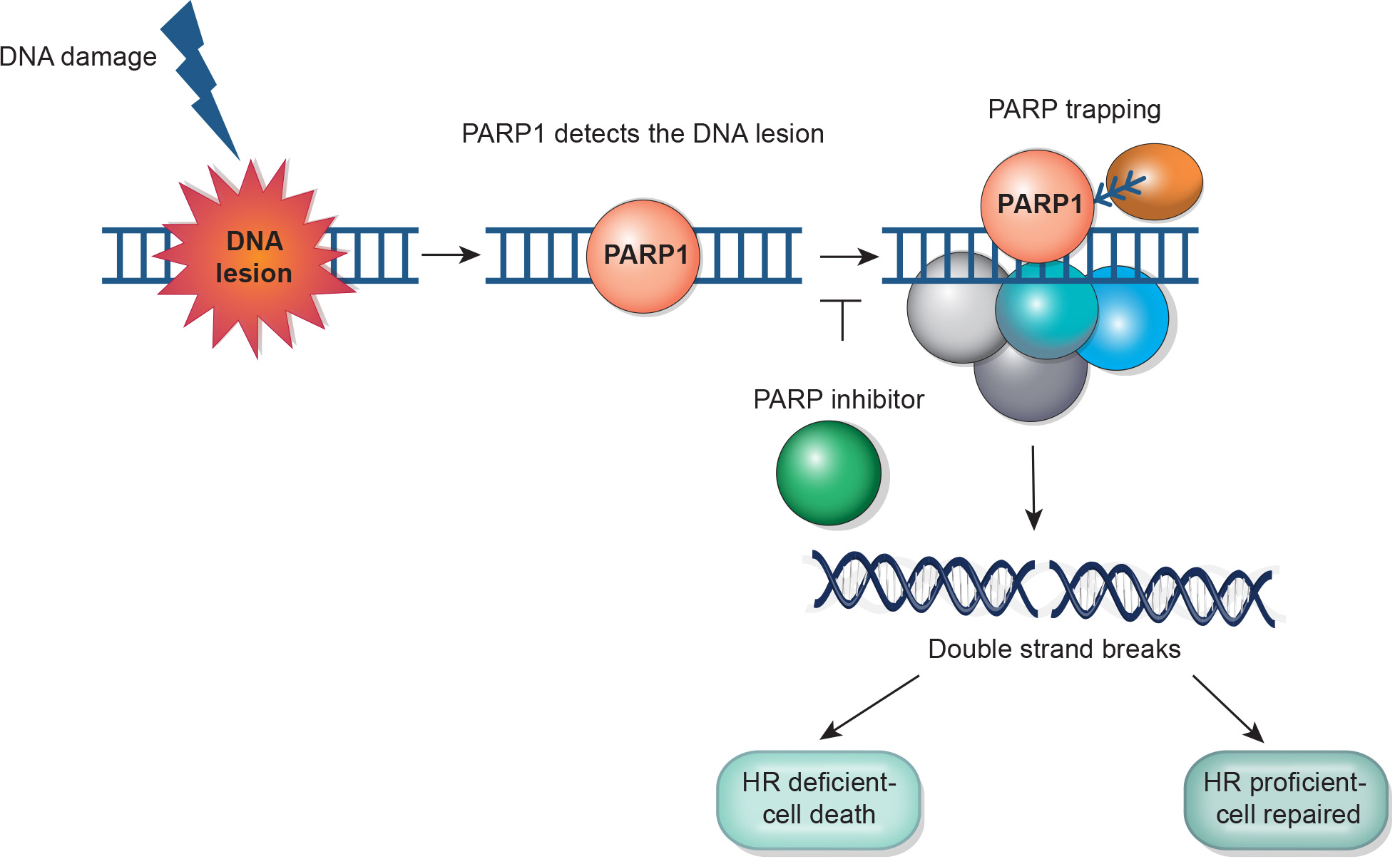
PARP Inhibitors in Prostate Cancer
PARPi are an emerging treatment for prostate cancer, particularly effective in men with metastatic castrate-resistant prostate cancer and specific homologous recombination repair deficiencies.
HR gene mutations are found in 10-15% (germline) and 20-25% (somatic) of metastatic prostate cancer cases, with BRCA2 and ATM being most common. PARPi research is expanding to earlier stages due to genetic alterations seen in localized disease, which confer a more aggressive course.
By inducing synthetic lethality, PARPi like olaparib and rucaparib have shown to improve tumor responses, progression-free survival, and overall survival. Olaparib is FDA approved for mCRPC patients with any HR mutation after a second-generation hormonal agent, while rucaparib is approved for those with BRCA1/2 mutations post-hormonal agent and taxane chemotherapy.
On August 11, 2023, the Food and Drug Administration approved the fixed dose combination of niraparib and abiraterone acetate (Akeega, Janssen Biotech, Inc.), with prednisone, for adult patients with deleterious or suspected deleterious BRCA-mutated castration-resistant prostate cancer (mCRPC)
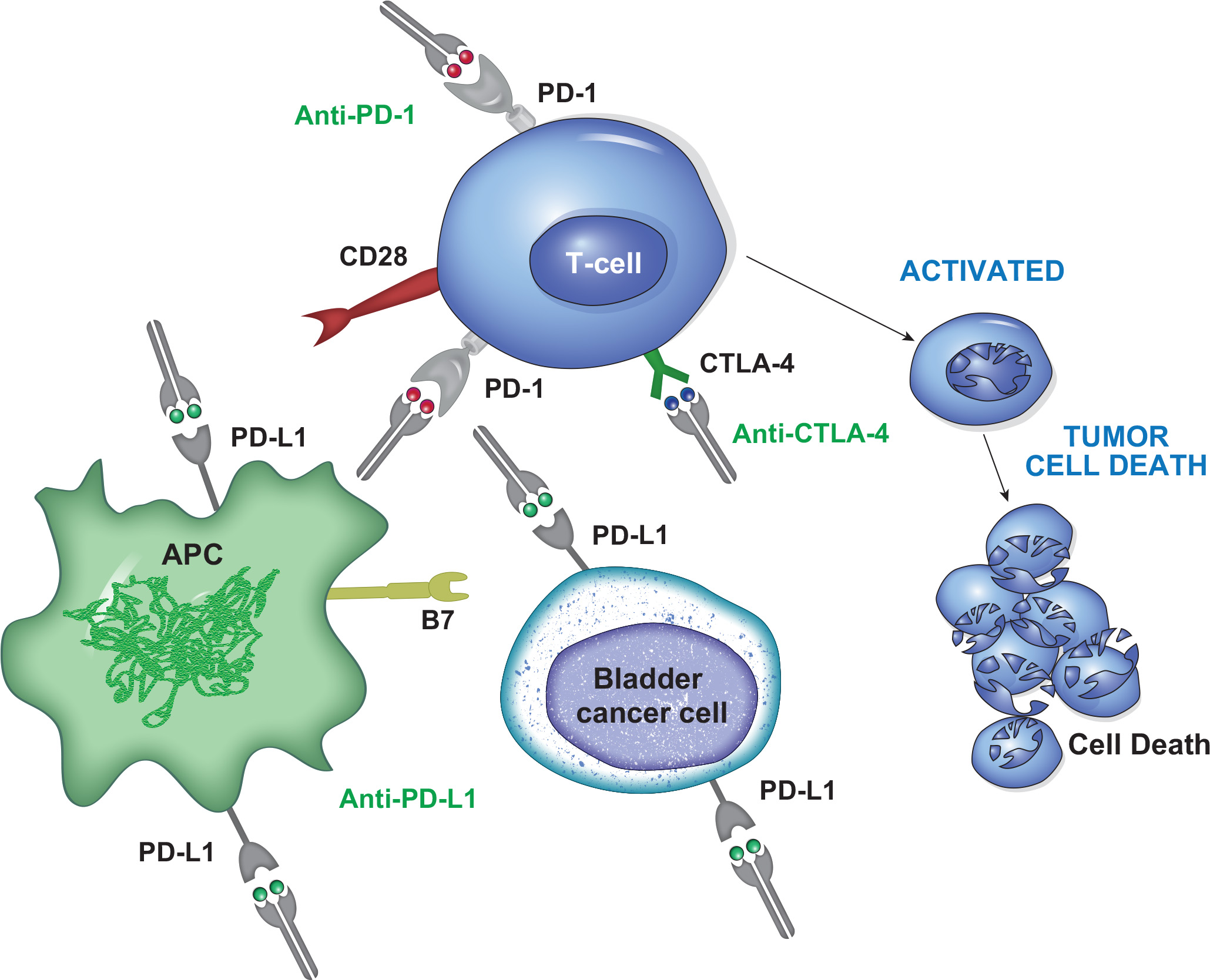
Checkpoint Inhibitors in Bladder Cancer
Several immune checkpoint inhibitors (ICIs) targeting PD-1 and PD-L1 have gained FDA approval for locally advanced or metastatic urothelial carcinoma.
These include pembrolizumab for platinum-refractory disease and as a first-line treatment for platinum-ineligible patients, nivolumab for platinum-refractory disease and as adjuvant therapy for high-risk surgical patients, and avelumab as switch-maintenance therapy for patients with stable disease post-platinum chemotherapy.
However, some patients do not respond to checkpoint inhibitors. As result, the capability for identifying patients that are eligible for this immunotherapy represent one of the efforts of ongoing studies.
On March 6, 2024, the Food and Drug Administration approved nivolumab (Opdivo, Bristol-Myers Squibb Company) in combination with cisplatin and gemcitabine for first-line treatment of adult patients with unresectable or metastatic urothelial carcinoma (UC)
On March 28, 2025, the Food and Drug Administration approved durvalumab (Imfinzi, AstraZeneca) with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent durvalumab as adjuvant treatment following radical cystectomy, for adults with muscle invasive bladder cancer (MIBC).
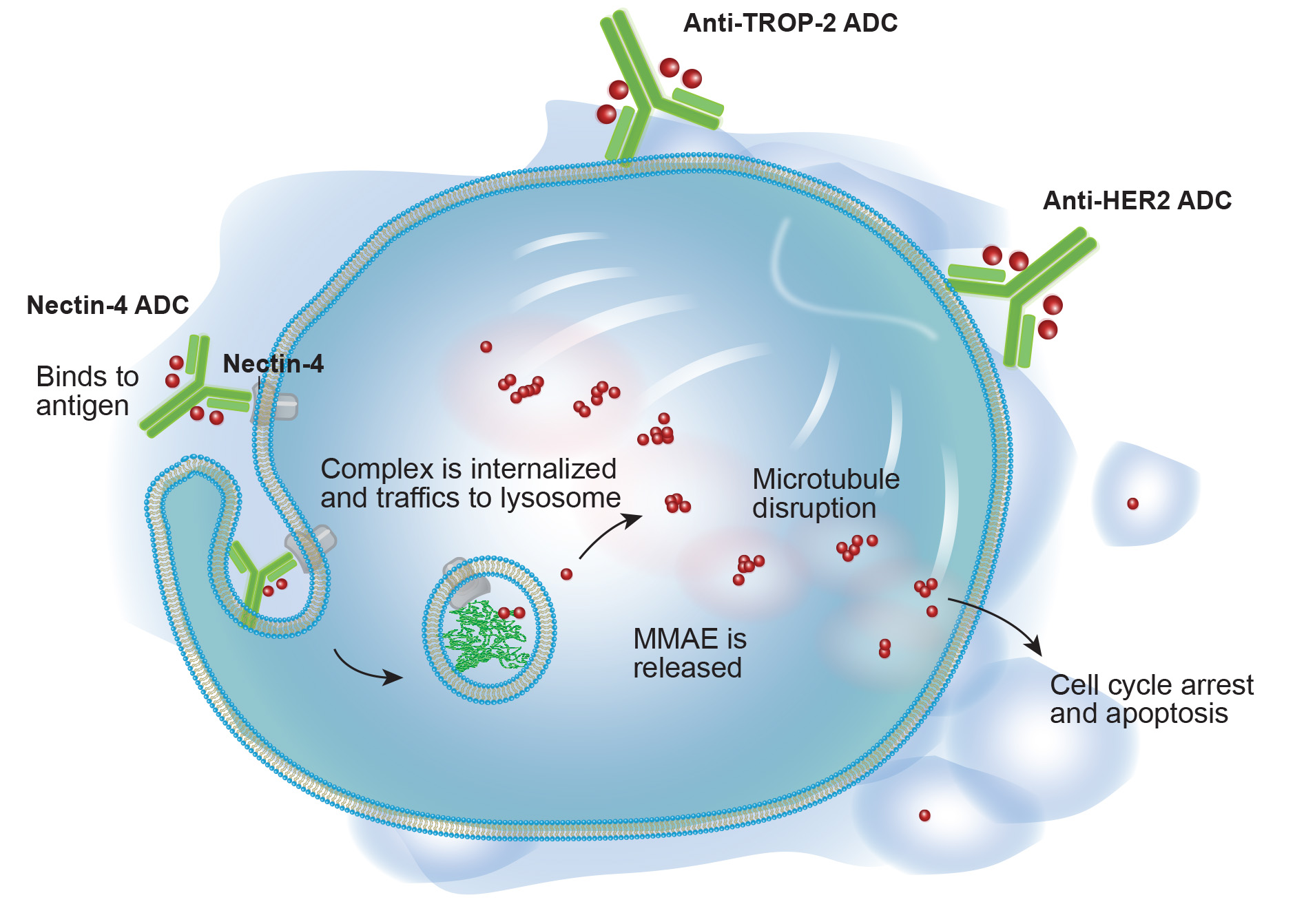
Antibody Drug Conjugates in Bladder Cancer
Current treatments for metastatic urothelial carcinoma include chemotherapy (GC or MVAC), immunotherapy (Pembrolizumab, Atezolizumab, or Avelumab), and targeted therapy (Erdafitinib for FGFR3 or FGFR2 mutations).
These treatments are limited by chemotherapy toxicities, low immunotherapy response rates, and the scarcity of relevant biomarkers.
Recently, novel therapies like antibody-drug conjugates (Enfortumab vedotin and Sacituzumab govitecan) have expanded treatment options and hold promise for improving outcomes in advanced UC.
On December 15, 2023, the Food and Drug Administration (FDA) approved enfortumab vedotin-ejfv (Padcev, Astellas Pharma) in combination with pembrolizumab (Keytruda, Merck) for patients with locally advanced or metastatic urothelial cancer (la/mUC).
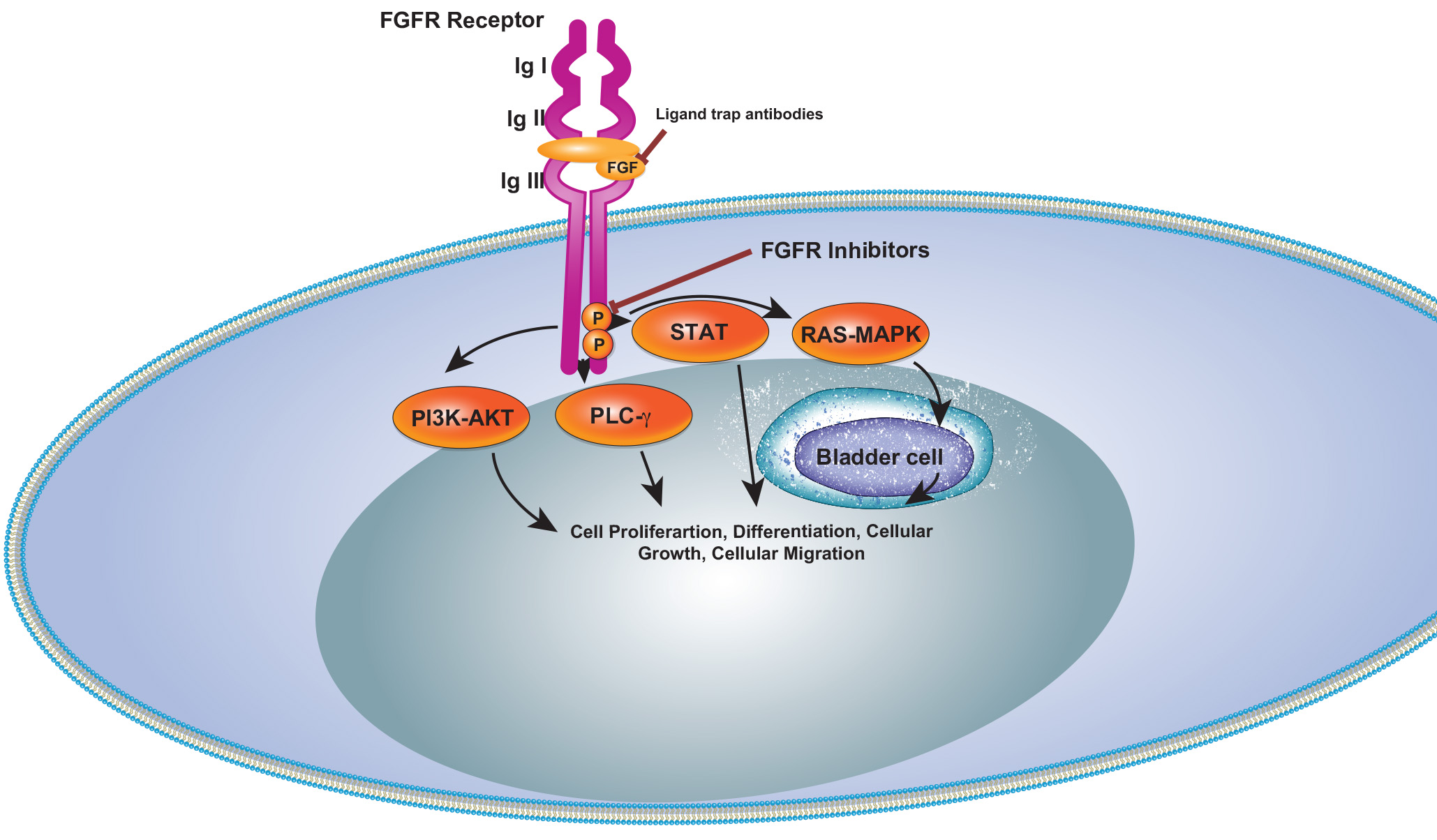
Targeting FGFR3 in Bladder Cancer
Fibroblast Growth Factor Receptors (FGFRs) have emerged as a novel therapeutic target in cancer. FGFR3 alterations, including activating mutations, fusions, and overexpression, are common in bladder cancer.
Up to 80% of non-muscle-invasive BC and 10-20% of muscle-invasive BC patients exhibit FGFR3 mutations or fusions, with FGFR3 overexpression in 40-50% of MIBC cases.
Erdafitinib, a pan-fibroblast growth factor receptor (FGFR) inhibitor, has been approved for treating patients with select FGFR2 and FGFR3 alterations and fusions since 2019. Since then, emerging data has demonstrated efficacy of combining erdafitinib with immunotherapy in treating FGFR-altered urothelial carcinoma.
On January 19, 2024, the Food and Drug Administration approved erdafitinib (Balversa, Janssen Biotech) for adult patients with locally advanced or metastatic urothelial carcinoma (mUC) with susceptible FGFR3 genetic alterations
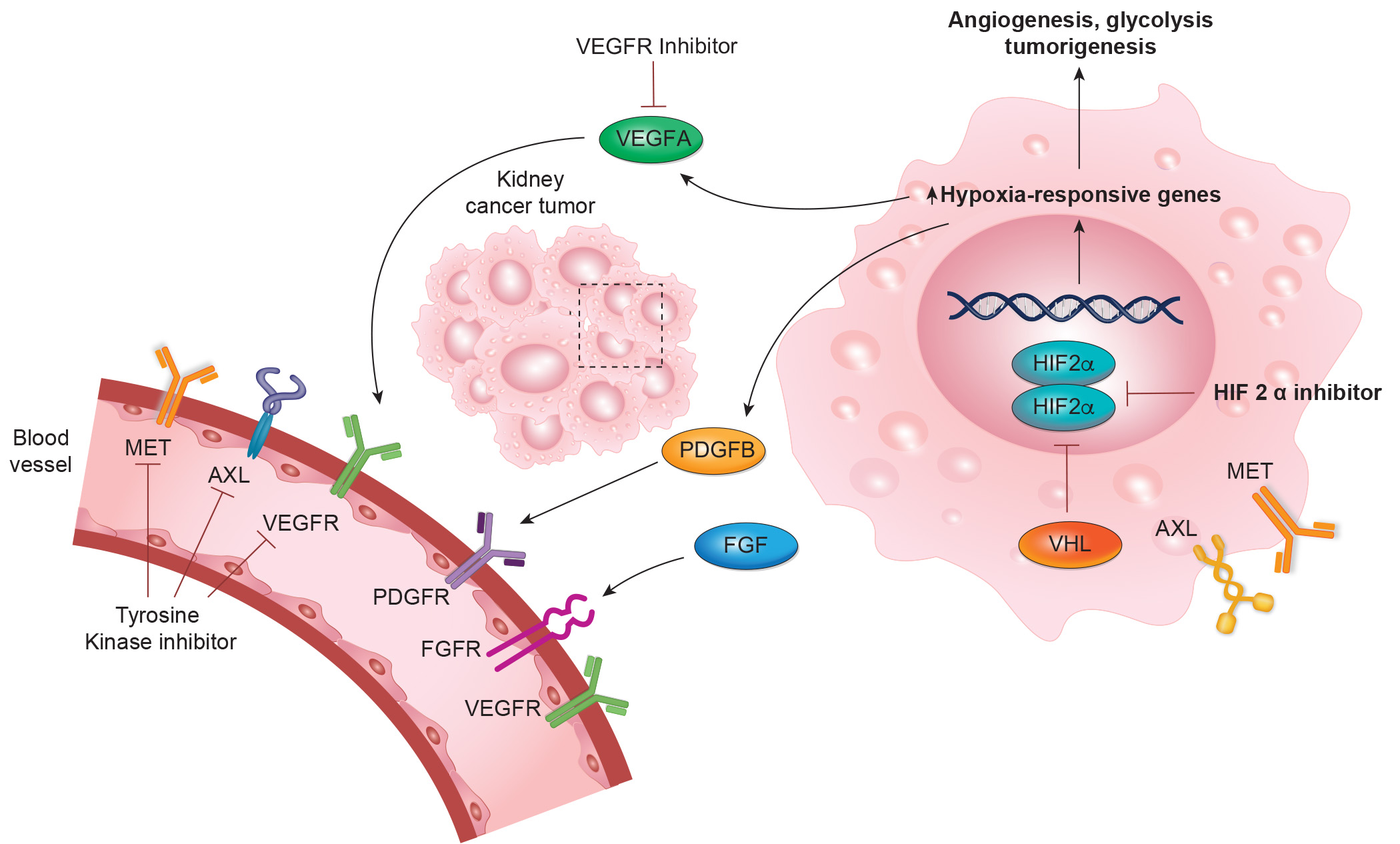
Targeting VEGF/HIF2 alpha in RCC
First-line therapies for mRCC include dual ICI combinations, tyrosine kinase inhibitors (TKIs) targeting VEGF, and combinations of ICIs with TKIs. Despite these advancements, the 5-year survival rate for mRCC remains around 15%.
Key VEGFR-TKIs include Axitinib, Cabozantinib, Sorafenib, Sunitinib, Pazopanib, Tivozanib, and Lenvatinib, along with the anti-VEGF monoclonal antibody bevacizumab.
Germline VHL mutations are central to hereditary VHL disease and VHL-associated RCC, with somatic VHL mutations in about half of sporadic clear cell RCC (ccRCC) cases. VHL gene function is uniformly lost in ccRCC with consequent accumulation of HIF-2α. HIF-2α inhibitors have entered the clinical arena as a new class of agents, alone and in combinations.
As of 2023, Belzutifan is the only FDA-approved HIF-2α inhibitor for both VHL-associated and sporadic RCC. On December 14, 2023, the Food and Drug Administration approved belzutifan (Welireg, Merck & Co., Inc.) for patients with advanced renal cell carcinoma (RCC) following a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI).